Specific Hazards including Atmospheric Hazards 1
A combination of hazards associated with the confined space regulations are addressed, which include the nature of the confined space; the possible presence of substances or conditions, which together make for an increased risk to the health and safety of people.
Bodycote HIP Ltd was fined £533,000 and ordered to pay costs of £200,000 on 24th July 2009 for breaching section 2(1) of the Health and Safety at Work etc Act 1974 following the deaths at its manufacturing plant in College Road, Hereford.
The court heard that on the 14th June, 2004, the company’s Works Manager and Maintenance Engineer were found collapsed on the stairs leading to a concrete-lined pit into which argon gas had leaked from a large pressure vessel. The pit’s oxygen alarm system was switched off and the ventilation system was not running.
HSE Inspector Luke Messenger said: “Both these tragic deaths were not only regrettable but also entirely preventable. The risks from confined spaces and asphyxiation due to the presence of argon were well known to the company, which had experience of a similar double fatality at a Bodycote Group site in California, just three years earlier.
“Despite this warning the company failed to undertake a proper risk assessment for entry into the confined space. Although they had implemented a safe system of work and permit to work procedure, they had not properly trained employees in their use, or ensured that these systems and procedures were being followed through their auditing procedure. On the day of the incident, the ventilation system, which could have removed the leaking argon before it became a problem, and the oxygen alarm system, which would have warned of the oxygen-depleted atmosphere, were not switched on. Had these systems been working these two deaths may not have occurred.”
Flammable substances and oxygen enriched atmospheres – Flammable gases occur naturally through organic decomposition of dirt and sludge. Alternatively, a flammable gas can be introduced by an external source. Additionally the risk of fire or an explosion can occur from the presence of flammable substances or an oxygen enriched atmosphere, i.e. leakage from an oxygen cylinder forming part of the welding equipment or leakages from adjourning plants or processes. Flammable gases are also produced by degradation of organic matter in soils which can be disturbed by working activities.
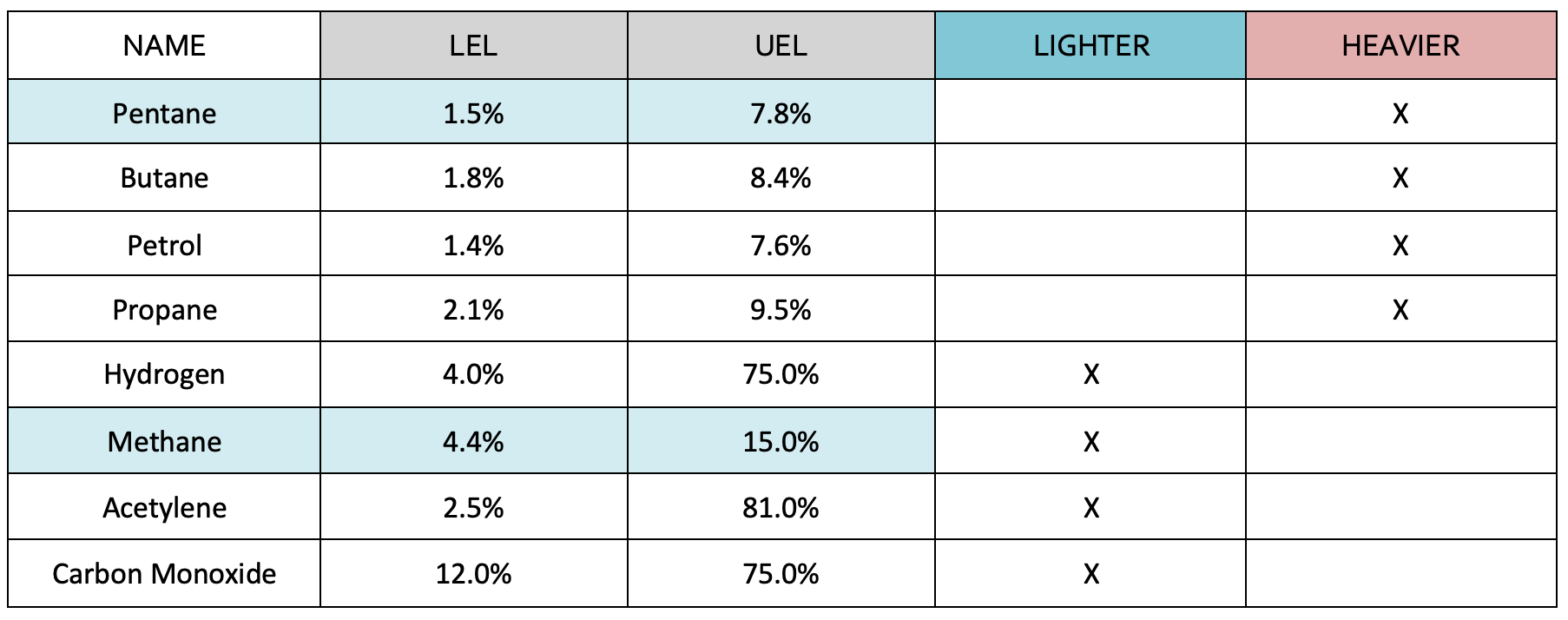
Examples of flammable gases include;
- Methane which is produced via sewage, rotting vegetation and natural gas
- Ammonia – refrigeration plants
- Petroleum – Spillages, residues, trade waste and road traffic incidents
- Acetylene – Welding operations
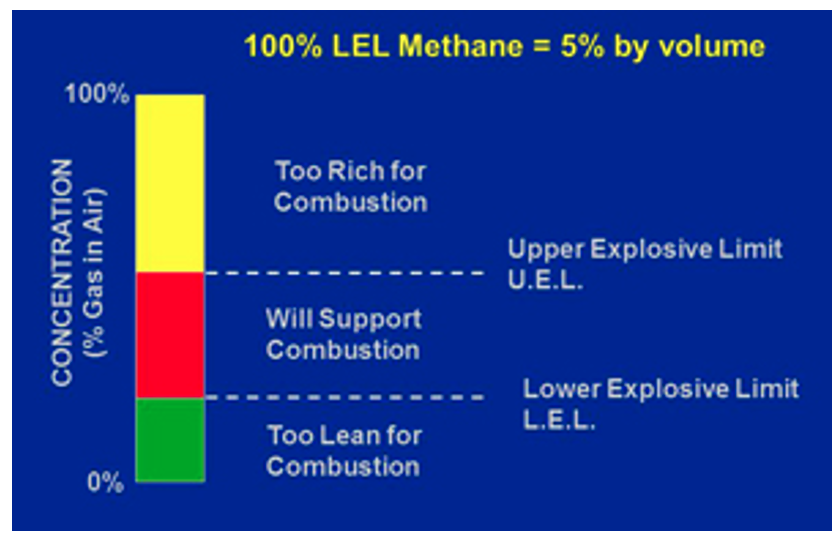
For a flammable atmosphere to become explosive, a mixture must be formed containing the appropriate amounts of fuel and oxygen. This term is known as its flammable range or limits of flammability. These terms are further explained below;
Lower Explosive Limit (LEL) is defined “in relation to a flammable contaminant, the concentration of the contaminant in air below which the propagation of a flame does not occur on contact with an ignition source.”
Upper Explosive Limit (UEL) is defined “in relation to a flammable contaminant, the concentration in air above which the propagation of a flame does not occur on contact with an ignition source.” Ideal Mixture or Stoichiometric Mixture is defined as the ideal mixture in which all the fuel and oxygen are used and produce the most aggressive and forceful reaction.
Dangerous Atmospheres (Flammable) – Problems associated with flammable atmospheres is that an explosion can occur when a flammable gas or vapour comes into contact with an ignition source such as; lighted cigarettes, matches, welding processes, static electricity, sparks, and hot embers including soot, electrical equipment, and naked lights. The power of the explosion will depend as previously mentioned where the flammable mixture lies between its flammable range.
Toxic Atmosphere – These can remain from previous processes or as a result from previous storage or from sludge or the disturbance of such deposits. Fumes can also enter from neighbouring plant systems or processes taking place inside the confined space, i.e. use of adhesives or solvents, welding equipment. However, some gases are produced naturally such as Hydrogen Sulphide H2S.
Hydrogen Sulphide is a colourless, heavier than air, very poisonous, flammable gas with the characteristic foul odour of rotten eggs. It often results from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestion. It also occurs in volcanic gases, natural gas, and some well waters. Large volumes of H2S destroy your sense of smell.
Due to the possible invisible hazard of a flammable/toxic atmosphere, in which the smallest amount of contaminates can have a catastrophic effect on the human body, they have to be detected very early.
Toxic gases are measured in parts per million (ppm) and 1% equates to 10,000ppm. The odour from rotten eggs has a range from 0.1 to around 200ppm.
Gas Detector alarms activate around depending upon manufacturer.
- Hydrogen Sulphide - 5ppm
- Carbon Monoxide - 30ppm
- Methane - 10% of its Lower Explosive Level
- Oxygen Levels - between 19.5% and 23.5%
- Chlorine - 2ppm
Chlorine is a toxic gas that irritates the respiratory system. Because it is heavier than air, it tends to accumulate at the bottom of poorly ventilated spaces. Chlorine gas is a strong oxidizer, which may react with flammable materials
Chlorine is detectable with measuring devices in concentrations of as low as 0.2 parts per million (ppm), and by smell at 3 ppm. Coughing and vomiting may occur at 30 ppm and lung damage at 60 ppm. About 1000 ppm can be fatal after a few deep breaths of the gas.[24] Breathing lower concentrations can aggravate the respiratory system, and exposure to the gas can irritate the eyes.
Carbon monoxide (CO), also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. When you breathe in carbon monoxide, it gets into your blood stream and prevents your red blood cells from carrying oxygen. Without oxygen, your body tissue and cells die. Levels that do not kill can cause serious harm to health when breathed in over a long period of time. Long term effects of carbon monoxide poisoning include Paralysis and brain damage.
An oxygen-deficient atmosphere (not enough oxygen in the space) has less than 19.5 percent of available oxygen. Any confined space with less than 19.5 percent oxygen should not be entered without an approved self-contained breathing apparatus.
The low oxygen level in a confined space can be caused by chemical reactions, sewage or other decomposing organic matter such as domestic waste and plant life. The work being done in the space or certain chemical reactions can also lower the oxygen level. In order to have a safe working environment in a confined space, the oxygen level must be between 19.5 and 23.5 percent. Any level below 19.5 percent is dangerous and will affect the worker’s health and safety. Levels below 10 percent can cause unconsciousness and levels below 8 percent can quickly cause death.
Ingress or presence of liquids Liquids can flow into the confined space, which may lead to drowning or other injuries depending upon the conditions.
Solid materials which can flow Free flowing solids can submerge a person and prevent breathing. Materials which create this hazard include grain, sugar, flour, sand, coal dust and or any other substance in a granular or powder form.
Presence of excessive heat This can lead to a dangerous rise in core body temperature which is further increased by the wearing of PPE.. Employers working in furnaces or boilers for example, the facility must be allowed to cool prior to entry.
A bakery company and three company directors were fined £373,000 after two workers died inside an oven. The men were carrying out maintenance work when they became trapped inside the machine where temperatures were above 100C. They died from burns and heat exposure after being unable to escape.
There was no system of reversing the conveyor and no system of getting them out of the oven.
Two engineers died after maintenance on the bread oven went wrong in May 1998. The men were sent into the oven only two hours after it had been switched off. They climbed in through the bottom and onto a conveyor used to take the dough through the giant machine. All they were wearing for protection were all-in-one suits.
The oven was normally set to run at 260C. It had only been turned off for two hours and the centre was 100C when these two men went inside. No-one, it seemed, looked at the temperature gauge at the side of the oven which would have indicated it wasn’t safe to go in. One engineer had taken a radio with him and after a few minutes inside sent a panicky message saying it was too hot.
They were trapped. There was no system of reversing the conveyor and no system of getting them out of the oven. It took 17 minutes - the time it took for the conveyor to pass through the oven - before other workers could help the men.
One engineer was brought out in “a state of collapse” and had extensive burns. The other was trapped inside the oven and had to be freed by the fire service. He had 80% burns to his body and died at the scene.